- Research article
- Open access
- Published:
Evidence of extensive cellular immune response after SARS-CoV-2 vaccination in ocrelizumab-treated patients with multiple sclerosis
Neurological Research and Practice volume 3, Article number: 60 (2021)
Abstract
Background
Patients with multiple sclerosis receiving ocrelizumab-treatment are in desperate need of a protection against SARS-CoV-2 infection.
Methods
In this study, Euroimmun semi-quantitative Anti-SARS-CoV-2 IgG for detection of humoral response and ELISpot assays for detection of SARS-CoV-2-specific T-cell-response were used in 10 ocrelizumab-treated patients with multiple sclerosis twice vaccinated with Comirnaty® mRNA vaccine. This data was compared with a control group of 20 age- and sex-matched healthy volunteers, who had all previously received a full SARS-CoV-2 mRNA vaccination with Comirnaty® or Spikevax®.
Results
While all subjects in the control group had high humoral response to the vaccination, in B-cell-depleted individuals a significantly reduced antibody response to vaccination against SARS-CoV-2 was observed. SARS-CoV-2 specific T-cell-response, however, did not differ significantly between both cohorts.
Conclusions
T-cell-mediated response to Comirnaty® vaccination is observable despite attenuated humoral response in B-cell-depleted patients. This might enable partial protection against COVID-19.
Trial registration Retrospectively registered.
Background
The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by infection with severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), is a major threat to global health and especially endangers individuals who suffer from chronic diseases such as multiple sclerosis (MS). While risk of hospitalization, intensive care and death due to COVID-19 in patients with multiple sclerosis (pwMS) is almost equal compared to healthy individuals [8, 17], patients undergoing disease modifying therapies and especially B-cell-depletion likely suffer from a more severe course of infection [9, 10, 16]. Sensitivity to more severe virus infections in B-cell-depleted patients is known to occur due to reduced humoral immunity, hampering effective virus clearance [12]. Hence, B-cell-depleted patients are in desperate need for protection against SARS-CoV-2 infection.
Another problematic aspect of B-cell-depletion is the compromised effectivity of vaccines, as demonstrated in the VELOCE study, where missing humoral response to various vaccines (such as tetanus and seasonal influenza vaccines) in ocrelizumab-treated patients was shown, leading to the recommendation to complete vaccination before initiation of ocrelizumab-therapy [4].
Therefore, the objective of this study was to investigate T-cell-response to SARS-CoV-2-vaccination in pwMS treated with ocrelizumab to gather information about humoral and cellular immunity in this fragile group of patients.
Methods
Volunteers
All patientsincluded in the study were recruited in the outpatient clinic for MS of the Alfried Krupp Hospital in Essen, Germany. All patients were treated with ocrelizumab (Ocrevus®) for MS; of these ten pwMS, eight had a relapsing remitting disease course and two were diagnosed with a primary progressive disease course. EDSS varied from 0 to 7. Ocrelizumab was assessed in a standard dose of 600 mg, but in terms of reducing social interaction of immunocompromised patients during COVID-19 pandemic, following cycles were not applied twice a year but only after B-cell-repopulation was detectable via FACS-analysis.
All patients included in this study were female. They had a mean age of 50 years (median 47, range 34–79). All received two vaccinations with Comirnaty® (BNT162b2, BioNTech/Pfizer). None reported symptoms of SARS-CoV-2 infection. The second vaccination was performed at a median of 34 days (range 25–46) prior to blood sampling.
The mean interval between last ocrelizumab cycle to first vaccination was 125 days (median 124, range 7–266) and to second vaccination 152 days (median 154, range 28–282), varying as many patients show long-term suppression of B-cells after ocrelizumab-application.
In most patients, B-cell-suppression was measured close to the time of vaccination. In three patients, B-cell count was 1% and in five patients, it was 0%. In two patients no FACS-analysis was performed, as ocrelizumab was applied shortly before vaccination and complete B-cell-suppression could be expected. In most patients (n = 6), FACS-analysis was performed between the two vaccinations, specifically 15 days (median 20, range 1–30) before the second vaccination. In one patient, FACS-analysis was done 8 days before first and 30 days before second vaccination. In another patient, FACS-analysis was sampled 28 days after second vaccination.
As a control group, we included 20 female healthy volunteers (mean age of 49 years, median 53, range 22–80), who had been vaccinated twice against SARS-CoV-2, without symptoms of SARS-CoV-2 infection before vaccination. Immunity of the vaccinated volunteers was analyzed at a median of 29 days (range 25–77) after the second vaccination. Seven of them were vaccinated with Comirnaty® (BioNTech/Pfizer) and 13 with Spikevax ® (mRNA-1273, Moderna Biotech).
As a further control for SARS-CoV-2 IgG antibodies, we tested 50 retention samples from our blood bank that were collected in November 2016.
The study was approved by the local ethics committee of the University Hospital Essen, Germany (20-9225-BO). All volunteers provided informed consent to participate in the study. This study has been performed in accordance with the ethical standards noted in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.
ELISpot assay
To assess SARS-CoV-2-specific cellular immunity, we performed ELISpot assays, using peptide pools of the Spike (S) 1, the S1/S2, the membrane (M) and the nucleocapsid (NC) protein (Miltenyi Biotec, Bergisch Gladbach, Germany) and an S1 protein of SARS-CoV-2 (S Sino, Sino Biological, Wayne, PA, USA). Whereas responses to the spike are expected after SARS-CoV-2 vaccination and infection, responses to the nucleocapsid should only occur after infection. We used this peptide pool to exclude volunteers with prior infection. Responses to the membrane are not completely specific for SARS-CoV-2 infection, but could also occur as a cross-reaction after infection with human endemic coronaviruses. We tested duplicates of 250,000 peripheral blood mononuclear cells (PBMC) per cell culture and measured IFN-γ production after 19 h. SARS-CoV-2 specific spots were determined as stimulated minus non-stimulated (background) values (spots increment). We defined at least three spots above background together with threefold higher SARS-CoV-2 specific spots versus background as positive response. Thereby, the detection limit of our assay is in the range of 3/250,000 functionally active and SARS-CoV-2 specific cells. Details on the ELISpot assay and the cutoff definition have been published previously [14].
Antibody ELISA
Antibodies were determined by a CE marked Anti-SARS-CoV-2 IgG semi-quantitative ELISA (Euroimmun, Lübeck, Germany), according to the manufacturers’ instructions. Results of S1 protein specific IgG antibodies are given as ratio (patient sample / control sample). An antibody ratio of > 1.1 was considered positive, of ≥ 0.8 to < 1.1 borderline and of < 0.8 negative.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 8.0.1 (San Diego, CA, USA). For the analysis of numerical variables, we used Spearman correlation and linear regression analysis. To assess the impact of categorical covariates we used Mann–Whitney test. Two-sided p values < 0.05 were considered significant.
Results
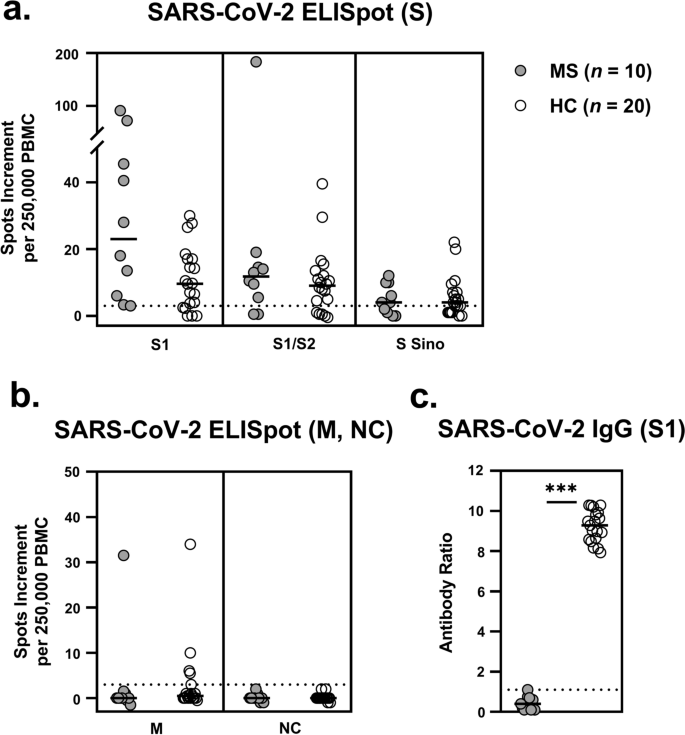
In all ten patients with multiple sclerosis who received the B-cell-depleting antibody ocrelizumab (Ocrevus®, anti-CD20 Ab) we observed—as expected—a significantly reduced antibody response to vaccination against SARS-CoV-2 (Fig. 1). The patients reached a median antibody ratio of 0.4 (range 0.1–1.1) and none of the responses could be classified as positive. In comparison, all 20 vaccinated, age- and sex-matched healthy controls showed detectable antibody responses, with a median S1-specific IgG ratio of 9.3 (range 7.9–10.3) (p < 0.0001). However, SARS-CoV-2 specific T cell responses did not differ significantly between both cohorts. In detail, ELISpot responses to the spike peptides S1 and S1/S2 were even slightly higher and to an S protein antigen (S Sino) were similar in patients with multiple sclerosis (S1: 23.0 vs. 9.6; S1/S2: 11.8 vs. 9.0; S Sino: 4.0 vs. 4.0, data represent median values of spots increment). Of note, also the 79 year-old female patient treated with Ocrelizumab developed strong T cell immunity. In the control experiments, none of the volunteers showed responses to the nucleocapsid, i.e., none had evidence of previous SARS-CoV-2 infection. Responses to a rather conserved coronavirus antigen, the membrane, were also similar in both cohorts.
SARS-CoV-2 specific ELISpot and IgG responses in patients with multiple sclerosis (MS) and matched healthy controls (HC). a ELISpot responses to spike (S), b to membrane (M) and nucleocapsid (NC) and c IgG antibody responses against S1. Horizontal bold lines indicate median values, dashed lines the cutoff for positive responses (3 spots increment or antibody ratio of 1.1). S1: peptide mix of the SARS-CoV-2 spike (S) 1; S1/S2: peptide mix of the spike (S) 1 and S2; S Sino: S1 protein; M: peptide mix of the membrane; NC: peptide mix of the nucleocapsid. ***p < 0.0001 (Mann–Whitney test)
Spearman analysis indicated that none of the SARS CoV-2 specific immune responses correlated significantly with age, the day after the second vaccination or the IgG ratio. There was even a trend for a negative correlation, when considering the S1 peptide mix (r = −0.47, p = 0.17) (Fig. 2). Of note, this peptide mix induced the strongest T-cell response. Interestingly, the difference between the patients and controls was most pronounced when using the S1 peptides as stimulus.
To find out if SARS-CoV-2 IgG antibodies were completely absent in the B-cell-depleted patients, we compared their antibody ratio with the ratio from 50 retention samples from our blood bank, that were collected in November 2016 (Fig. 3). Of note, the median antibody ratio in the patients was more than twofold higher (0.40 vs. 0.17, p = 0.08). However, this finding reached no statistical significance.
Discussion
The current study indicates that B-cell-depletion in pwMS did not impair cellular immune responses after SARS-CoV-2 mRNA vaccination. Although all S1-specific IgG antibodies were below or at the cutoff for positive responses, they were higher than in retention samples from 2016.
As published recently, only 10 of 44 individuals (22.7%) of an Israeli cohort of pwMS receiving ocrelizumab-treatment developed humoral response to COVID-19 vaccine Comirnaty [1]. As individuals with B-cell repopulation had proper humoral response, postponement of the next therapy cycle in favour of an immune response to the COVID-19-vaccination has been recommended [3], eventually risking a higher occurrence of relapse in pwMS, especially since ocrelizumab is commonly prescribed in patients with highly active disease.
T-cell-mediated immunity might bypass such a dilemma because it is largely unaffected by CD19-depletion. As proof of principle, VZV-specific T-cell-response to VZV-vaccination was detected in patients receiving B-cell-depletion with rituximab in haematological diseases [13]. In 2017, Zhao et al. [18] already described the importance of T-cells for the recovery from a structurally related coronavirus, the Middle East respiratory syndrome (MERS) virus. In 2020, Braun et al. [7] speculated that T-cell immunity could also be protective against infection with SARS-CoV-2. Furthermore, SARS-CoV-2-specific T-cell-response was measured in antibody-seronegative family members of SARS-CoV-2-infected individuals [15]. There are now numerous publications confirming the importance of T-cell-responses for protection against SARS-CoV-2 [2, 6, 11].
It needs to be analyzed in a larger cohort of MS patients whether the strong T cell response just occurred randomly or whether it is a kind of compensation for the weak antibody response. We observed in approximately 15–20% of healthy controls only SARS-CoV-2 specific B or T cell immunity, despite PCR confirmed infection [14]. Thus, the immune response may be shifted more to an antibody or cellular response also in healthy individuals.
Conclusion
In conclusion, this is the first published finding showing that, despite B-cell depletion due to Ocrelizumab treatment, pwMS who have been fully vaccinanated with Comirnaty® still show an extensive SARS-CoV 2 specific T-cell response. Similar data was recently shown in five patients treated with rituximab in rheumatic diseases [5]. Moreover, specific B-cell immunity was not completely absent and may provide some protection. However, this study has some limitations, specifically low number of patients and different intervals between Ocrelizumab and vaccination. Nevertheless, we suggest that vaccination under B-cell-depletion should be favored over delaying treatment cycles of ocrelizumab thus risking relapse after B-cell-repopulation.
Availability of data and materials
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Acute respiratory syndrome coronavirus type 2
- MS:
-
Multiple sclerosis
- pwMS:
-
Patients with multiple sclerosis
- EDSS:
-
Expanded Disability Status Scale
- PBMC:
-
Peripheral blood mononuclear cells
- FACS-analysis:
-
Fluoresecence activated cell sorting
- ELISpot:
-
Enzyme Linked Immuno Spot Assay
- ELISA:
-
Enzyme-linked Immunosorbent Assay
References
Achiron, A., Mandel, M., Dreyer-Alster, S., Harari, G., Magalashvili, D., Sonis, P., Dolev, M., Menascu, S., Flechter, S., Falb, R., & Gurevich, M. (2021). Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Therapeutic Advances in Neurological Disorders, 14, 17562864211012836. https://doi.org/10.1177/17562864211012835
Altmann, D. M., & Boyton, R. J. (2020). SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Science Immunology. https://doi.org/10.1126/sciimmunol.abd6160
Baker, D., Roberts, C. A., Pryce, G., Kang, A. S., Marta, M., Reyes, S., Schmierer, K., Giovannoni, G., & Amor, S. (2020). COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clinical & Experimental Immunology, 202(2), 149–161. https://doi.org/10.1111/cei.13495
Bar-Or, A., Calkwood, J. C., Chognot, C., Evershed, J., Fox, E. J., Herman, A., Manfrini, M., McNamara, J., Robertson, D. S., Stokmaier, D., & Wendt, J. K. (2020). Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology, 95(14), e1999–e2008. https://doi.org/10.1212/wnl.0000000000010380
Bonelli, M. M., Mrak, D., Perkmann, T., Haslacher, H., & Aletaha, D. (2021). SARS-CoV-2 vaccination in rituximab-treated patients: Evidence for impaired humoral but inducible cellular immune response. Annals of the Rheumatic Diseases. https://doi.org/10.1136/annrheumdis-2021-220408
Bonifacius, A., Tischer-Zimmermann, S., Dragon, A. C., Gussarow, D., Vogel, A., Krettek, U., Gödecke, N., Yilmaz, M., Kraft, A. R., Hoeper, M. M., & Pink, I. (2021). COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity, 54(2), 340-354.e346. https://doi.org/10.1016/j.immuni.2021.01.008
Braun, J., Loyal, L., Frentsch, M., Wendisch, D., Georg, P., Kurth, F., Hippenstiel, S., Dingeldey, M., Kruse, B., Fauchere, F., & Baysal, E. (2020). SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature, 587(7833), 270–274. https://doi.org/10.1038/s41586-020-2598-9
Fernandes, P. M., O’neill, M., Kearns, P. K., Pizzo, S., Watters, C., Baird, S., MacDougall, N. J., & Hunt, D. P. (2020). Impact of the first COVID-19 pandemic wave on the Scottish Multiple Sclerosis Register population. Wellcome Open Research, 5, 276. https://doi.org/10.12688/wellcomeopenres.16349.1
Landtblom, A. M., Berntsson, S. G., Boström, I., & Iacobaeus, E. (2021). Multiple sclerosis and COVID-19: The Swedish experience. Acta Neurologica Scandinavica. https://doi.org/10.1111/ane.13453
Langer-Gould, A., Smith, J. B., & Li, B. H. (2021). Multiple sclerosis, rituximab, and COVID-19. Annals of Clinical and Translational Neurology, 8(4), 938–943. https://doi.org/10.1002/acn3.51342
Le Bert, N., Tan, A. T., Kunasegaran, K., Tham, C. Y., Hafezi, M., Chia, A., Chng, M. H., Lin, M., Tan, N., Linster, M., & Chia, W. N. (2020). SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature, 584(7821), 457–462. https://doi.org/10.1038/s41586-020-2550-z
Misumi, I., & Whitmire, J. K. (2014). B cell depletion curtails CD4+ T cell memory and reduces protection against disseminating virus infection. Journal of Immunology, 192(4), 1597–1608. https://doi.org/10.4049/jimmunol.1302661
Parrino, J., McNeil, S. A., Lawrence, S. J., Kimby, E., Pagnoni, M. F., Stek, J. E., Zhao, Y., Chan, I. S., & Kaplan, S. S. (2017). Safety and immunogenicity of inactivated varicella-zoster virus vaccine in adults with hematologic malignancies receiving treatment with anti-CD20 monoclonal antibodies. Vaccine, 35(14), 1764–1769. https://doi.org/10.1016/j.vaccine.2016.10.055
Schwarzkopf, S., Krawczyk, A., Knop, D., Klump, H., Heinold, A., Heinemann, F. M., Thümmler, L., Temme, C., Breyer, M., Witzke, O., & Dittmer, U. (2021). Cellular IMMUNITY in COVID-19 convalescents with PCR-confirmed infection but with undetectable SARS-CoV-2-specific IgG. Emerging Infectious Diseases, 27(1), 66. https://doi.org/10.3201/2701.203772
Sekine, T., Perez-Potti, A., Rivera-Ballesteros, O., Strålin, K., Gorin, J. B., Olsson, A., Llewellyn-Lacey, S., Kamal, H., Bogdanovic, G., Muschiol, S., & Wullimann, D. J. (2020). Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell, 183(1), 158-168.e114. https://doi.org/10.1016/j.cell.2020.08.017
Sormani, M. P., De Rossi, N., Schiavetti, I., Carmisciano, L., Cordioli, C., Moiola, L., Radaelli, M., Immovilli, P., Capobianco, M., Trojano, M., & Zaratin, P. (2021). Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Annals of Neurology, 89(4), 780–789. https://doi.org/10.1002/ana.26028
Zabalza, A., Cárdenas-Robledo, S., Tagliani, P., Arrambide, G., Otero-Romero, S., Carbonell-Mirabent, P., Rodriguez-Barranco, M., Rodríguez-Acevedo, B., Restrepo Vera, J. L., Resina-Salles, M., & Midaglia, L. (2020). COVID-19 in multiple sclerosis patients: Susceptibility, severity risk factors and serological response. European Journal of Neurology. https://doi.org/10.1111/ene.14690
Zhao, J., Alshukairi, A. N., Baharoon, S. A., Ahmed, W. A., Bokhari, A. A., Nehdi, A. M., Layqah, L. A., Alghamdi, M. G., Al Gethamy, M. M., Dada, A. M., & Khalid, I. (2017). Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Science Immunology, 2(14), 66. https://doi.org/10.1126/sciimmunol.aan5393
Acknowledgements
We would like to thank all the patients who participated. We would also like to thank Christine Timm and Babette Große-Rhode for perfect assistance in recruitment and management of patients. We are thankful for English language support to Dr. Michèle Herold, Gelnhausen. Moreover, we appreciate good cooperation with Dr. Yuriko Stiegler and Dr. Hugo Stiegler, MVZLM laboratory, Essen, Germany.
Author information
Authors and Affiliations
Contributions
MP drafted the manicript. NF and ML perfomed the analysis, PH and MK corrected the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee of the University Hospital Essen, Germany (20-9225-BO). All volunteers provided informed consent to participate in the study. This study has been performed in accordance with the ethical standards noted in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
All patients agreed for publication.
Competing interests
MP, NF, PAH and ML declare that there is no conflict of interest. MK received honoraria for teaching activities from Roche Pharma and Chugai Pharma. Funding: There was no funding.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pompsch, M., Fisenkci, N., Horn, P.A. et al. Evidence of extensive cellular immune response after SARS-CoV-2 vaccination in ocrelizumab-treated patients with multiple sclerosis. Neurol. Res. Pract. 3, 60 (2021). https://doi.org/10.1186/s42466-021-00158-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42466-021-00158-5